Abstract
Introduction: Novel triplet regimens containing immunomodulatory drugs and proteasome inhibitors (PIs) have improved outcomes and extended survival for patients (pts) with RRMM. However, use of combination therapies (tx) over an extended period may increase tx-related symptom burden and impact health-related quality of life (HRQoL). In the randomized phase 2 ELOQUENT-3 trial (NCT02654132), the efficacy and safety of elotuzumab (ELO) + pomalidomide + dexamethasone (EPd) vs pomalidomide + dexamethasone (Pd) were assessed in pts with RRMM previously treated with lenalidomide (LEN) and a PI. EPd had a 46% reduction in the risk of death vs Pd, without increasing toxicity or affecting HRQoL through 18.3 months (mo) follow-up (f/u) (Dimopoulos MA, et al. N Engl J Med 2018;379:1811-1822; Weisel K, et al. Blood 2019;134[suppl 1];Abstract 3480). To inform tx decision-making, it is important to measure how effective tx can preserve HRQoL without a negative impact. Here we evaluate updated HRQoL findings from ELOQUENT-3 after 4 years of EPd tx (data cutoff, February 2021), including the first report of responder analyses.
Methods: Pt-reported outcomes (PROs) were exploratory endpoints, assessed using the EuroQoL 5 dimension 3 level (EQ-5D-3L) instrument and the MD Anderson Symptom Inventory MM module (MDASI-MM). EQ-5D-3L incorporates a global health visual analog scale (VAS; score range 0-100) and a descriptive system consisting of 5 dimensions to which UK empirically derived weights were applied to generate a utility index (UI; score range −0.59 to −1); MDASI-MM measures total symptom severity (20 items) and symptom interference (6 items) (score range 0-10). Minimally important differences (MIDs)/responder definitions were 0.08 (UI), 7 (VAS), or based on the standard error of the mean (MDASI-MM). Higher scores indicate better health for EQ-5D-3L, but more severe symptoms for MDASI-MM. PRO data were collected at baseline (BL), the start of every 28-day tx cycle, the end of tx, and during f/u every 3 mo. All randomized pts with BL and ≥ 1 post-BL assessment were included in the PRO analysis. Completion rates are presented out of the expected population. Changes from BL were evaluated descriptively and using a mixed model for repeated measures. Time to first deterioration was defined as time to first change from BL that was at least the responder definition threshold; hazard ratios (HR) were calculated using a Cox proportional hazard model. Responder analyses present the proportion of improved/stable/worsened pts at each time point based on responder definition.
Results: Of 117 randomized pts, 106 (EPd n=55; Pd n=51) were included in PRO analyses. BL characteristics of the PRO population were balanced between arms and representative of the entire study population. Except for 2 cycles, PRO completion rates were ≥ 80% for both PROs for all on-tx time points where ≥ 10 pts remained on study. Although 11 pts remained on EPd until cycle 29, between-tx arm HRQoL analysis was not feasible after cycle 13 due to low Pd pt numbers. After end of tx, f/u PRO data were available for a longer period for EPd vs Pd (for ≥ 10 pts up to f/u visit 6 (18 mo) for EPd and up to f/u visit 2 (6 mo) for Pd).
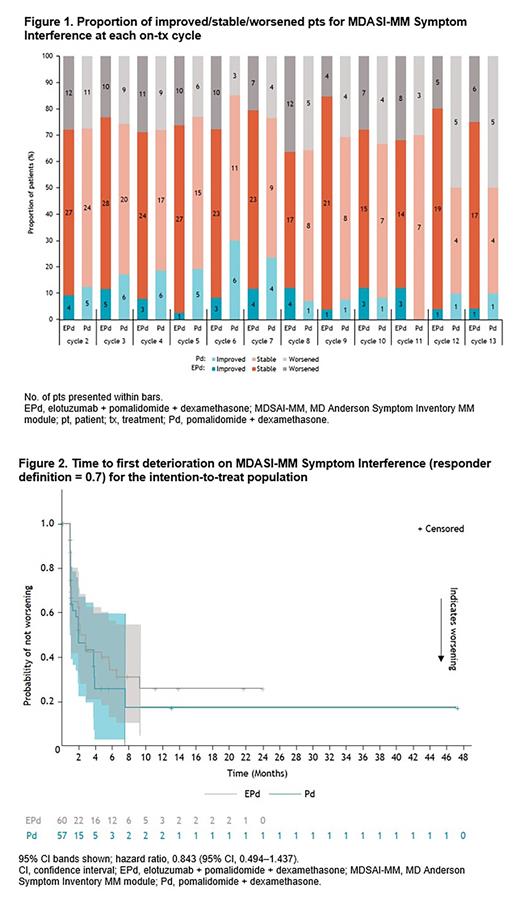
Mean BL scores were similar between EPd and Pd arms, and least squares mean change from BL data showed no overall clinically meaningful differences between tx arms for EQ-5D-3L UI or VAS, or MDASI-MM total symptom severity, symptom interference, or items of pain, fatigue, or bone pain. For the EPd tx arm, the proportion of pts who either improved or maintained stable HRQoL based on responder analyses up until cycle 13 ranged 82%-96% for MDASI total symptom score and 64%-85% for MDASI symptom interference (Fig. 1). There were no statistically significant differences in time to first deterioration between arms for key EQ-5D-3L and MDASI-MM scales. Kaplan-Meier plots presenting f/u data up to 48 mo were overlapping, indicating no HRQoL differences between EPd and Pd (Fig. 2).
Conclusions: HRQoL was similar between pts who received triplet EPd and doublet Pd in ELOQUENT-3, thus the addition of ELO to Pd did not significantly impact time to deterioration in HRQoL over an extended f/u period. These pt-reported findings complement existing f/u data showing that EPd tx resulted in clinically meaningful improvements in survival without detriment to HRQoL, further supporting the clinical value of EPd in adult pts with RRMM who have received ≥ 2 prior tx including LEN and a PI.
Weisel: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Novartis: Honoraria; Pfizer: Honoraria. Dimopoulos: BMS: Honoraria; Takeda: Honoraria; Beigene: Honoraria; Janssen: Honoraria; Amgen: Honoraria. San-Miguel: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Paner: Adaptive Biotechnologies: Consultancy; Amgen: Consultancy; GSK: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Oncopeptides: Consultancy; Rush University Medical Center: Consultancy, Current Employment; Sanofi: Consultancy; BMS: Consultancy. Taylor: Adelphi Values: Current Employment; BMS: Consultancy. Lord-Bessen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Yao: BMS: Current Employment. Yip: Adelphi Values: Current Employment. Greenwood: Adelphi Values: Current Employment. Tang: BMS: Consultancy. Cavo: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau; Novartis: Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal